The World of Biosecurity and Biosafety: An Overview
By Chris Isaac
Member of the Lethbridge team 2012 - 2019 and Advisor to the Lethbridge HS team from 2015 to 2019
Via Unspalsh - Chris Brignola
No country has been able to prevent SARS-CoV-2 from entering its borders. The COVID-19 pandemic is a global issue that has and will continue to disproportionately affect poor nations and disadvantaged communities. Even as countries scramble to contain the virus, the pandemic continues to create economic damage, harm to human health, and loss of life several months after the disease was first identified. Though naturally occuring, SARS-CoV-2 highlights the devastating risks posed by biology, and provides a striking reminder that scientists and synthetic biologists have a clear duty to prevent mistakes and misuse of our craft.
As iGEMers, we participate in the iGEM Competition because we believe that synthetic biology is going to change the world. By harnessing the power of biology, we are attempting to disrupt conventional business and manufacturing practices and create new value in medical therapeutics and diagnostics, energy and environment, food and nutrition, and developing new technologies to unlock the full potential of living systems. We hope that a future based on biological innovation will be sustainable, safe, and just. However, it is clear that the road to that future is long, uneven, and winding. Recent events showcase how much more work there is to be done across social, political, and technical areas.
Following the outbreak of the original SARS coronavirus and the subsequent epidemic from 2002-2004, the World Health Organization (WHO) revised the International Health Regulations (IHR) in 2005. The purpose of the IHR is to strengthen countries against public health emergencies and provide a legally-binding framework by which the 196 states-parties to the WHO resolve to prevent, protect against, control, and provide a public health response to the international spread of disease. Despite the progress made, COVID-19 has laid bare the inadequacy of the global effort to prevent the emergence of pandemic diseases.
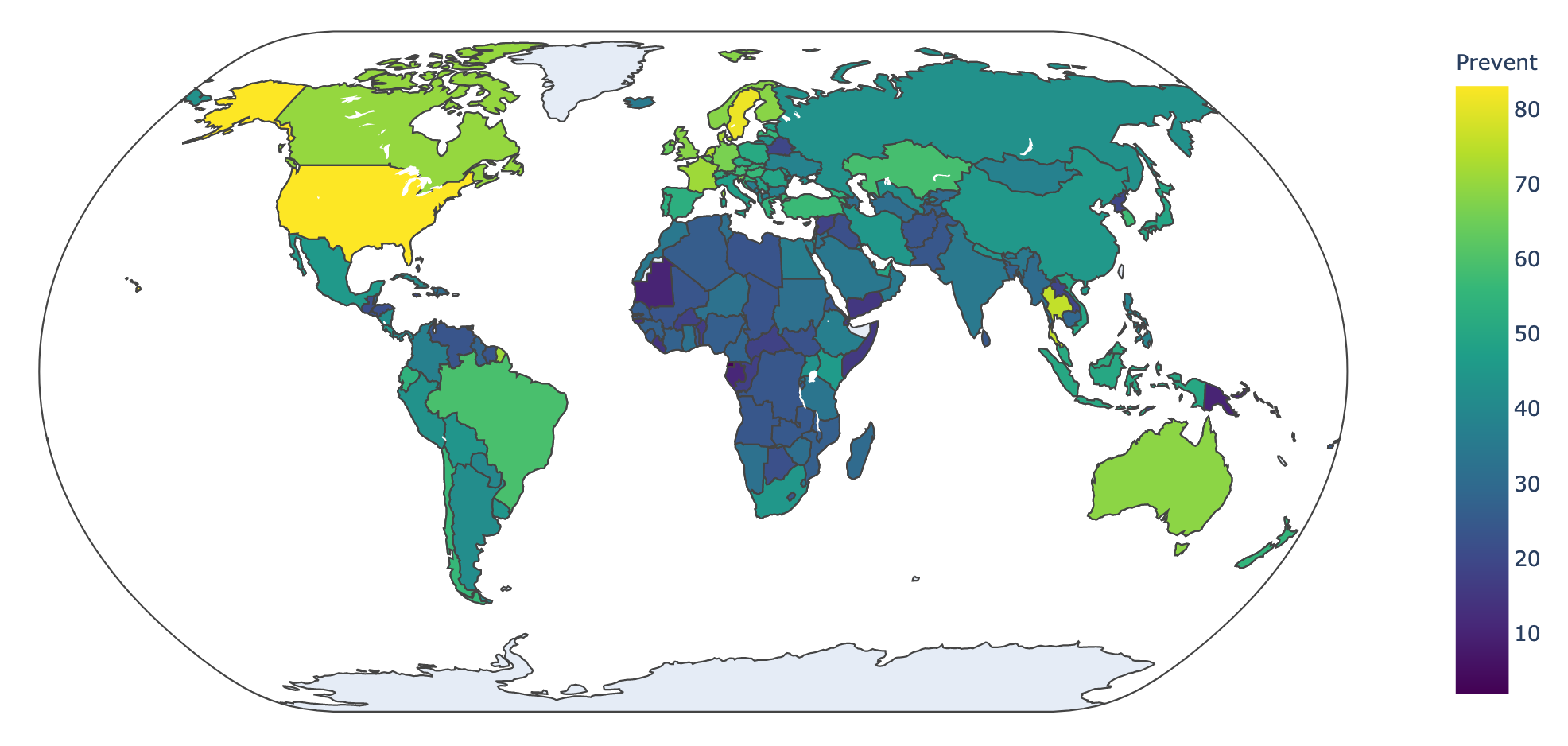
The Global Health Security Index* (2019) assessed the global health security capacities of 195 countries using open-source data. The average overall score for the world was a failing 40.2 out of a possible 100. Within the category of prevention, the average score was lower still: 34.8. In this article, we will provide a global overview of prevention-related indicators that are near and dear to iGEM: biosafety, which is the practice of protecting lab workers, the public, and the environment from infectious agents; biosecurity, which is the practice of preventing infectious agents from loss, theft, or misuse; dual-use, which are technologies that can be used for good and bad purposes; antimicrobial resistance, which is the accumulation of resistance factors in pathogenic organisms; and DNA synthesis, which is the assembly of nucleotides into meaningful sequences and the backbone of modern synthetic biology.
Globally, standards for biosafety and biosecurity are not as thoroughly woven into national policy as they are into the iGEM Competition. Although variation exists within the category of prevention (Figure 1), it is apparent that no country is fully prepared to prevent a pandemic and that a gap in preparedness anywhere is a risk everywhere. Without national oversight, it falls to scientists to conduct their research safely and securely. As synthetic biologists are already engaging with issues of biosafety and biosecurity, it is critical for iGEMers to maintain an ethos of responsible science and carry it into the research cultures of their home nations.
Figure 1: There are 196 countries that are party to the International Health Regulations. The Global Health Security Index assesses 195 countries who are party to the IHR. There are teams in iGEM representing 45 countries (in 2019), but there are 0 countries that are fully prepared to prevent the emergence of novel diseases. The color of each nation represents their cumulative score in the category of Prevention.
National Agencies of Biosecurity Legislation
The structure of an organization or institution speaks to its priorities. The presence of national agencies focused on “bio” issues indicates the acknowledgement of biosafety and biosecurity as important facets of a cohesive health security strategy. In particular, the GHS Index assesses countries on the presence or absence of an agency with the mission of preventing potentially dangerous organisms from going missing or falling into the wrong hands. Biosecurity practices are designed to minimize the risk that an organism is deliberately misused.
Figure 2: (GHS Index 1.3.1c) The Index asks: “Is there an established agency (or agencies) responsible for the enforcement of biosecurity legislation and regulations?”
According to Figure 2, overall, there are very few national agencies focused on ensuring the security of infectious organisms. The countries that have biosecurity agencies are: Australia, Bulgaria, Canada, Chile, China, Cuba, Czech Republic, Denmark, Estonia, France, Georgia, Hungary, Indonesia, Ireland, Israel, Japan, Netherlands, New Zealand, Norway, Singapore, Slovakia, South Korea, Sweden, Thailand, Ukraine, United Kingdom, and the United States.
Biosafety Training
iGEMers, alumni or prospective participants, should know that working safely is a principal requirement of the iGEM process. The iGEM Rules of Conduct demand that teams assess and manage any and all risks associated with their project. iGEM also provides a detailed set of pages on the competition wiki with links to resources to help teams work safely and securely. Teams are also forbidden from working with especially dangerous pathogens. However, when it comes to national regulations regarding the most dangerous pathogens, there are very few countries that mandate standardized biosafety training.
Figure 3: (GHS Index 1.4.2a) The Index asks, “Does the country require biosafety training, using a standardized, required approach, such as through a common curriculum or a train-the-trainer program, for personnel working in facilities, housing, or working with especially dangerous pathogens, toxins, or biological materials with pandemic potential?”
As seen in Figure 3, the countries that require standardized biosafety training are: Armenia, Belgium, Canada, Chile, Denmark, Ecuador, Finland, France, Georgia, Germany, Greece, Kazakhstan, Kenya, Netherlands, Norway, Singapore, Sweden, Slovenia, Thailand, Turkey, Uganda, and the United States.
Biosecurity Training
Similarly, national requirements for biosecurity training are few and far between. Only Azerbaijan, Canada, Denmark, Germany, Kenya, Norway, Singapore, Sweden, Thailand, Turkmenistan, and the United States have mandated and standardized training to reduce risks from deliberate misuse of dangerous pathogens. Though many countries do not actively engage in research on especially dangerous pathogens, it is important that countries have plans to protect potentially infectious samples from patients with transmissible diseases like Ebola, influenza, or SARS-CoV-2.
Figure 4: (GHS Index 1.3.2a) The Index asks, “Does the country require biosecurity training, using a standardized, required approach, such as through a common curriculum or a train the-trainer program, for personnel working in facilities housing or working with especially dangerous pathogens, toxins, or biological materials with pandemic potential?”
According to Figure 4: Standardized and mandatory biosecurity training is required in: Azerbaijan, Canada, Denmark, Germany, Kenya, Norway, Singapore, Sweden, Thailand, Turkmenistan, and the United States.
Dual-Use Research of Concern
The desire to develop new technologies is at the heart of iGEM. But the development of new technologies can present the opportunity for promise and peril. For example, while research into designing proteins that effectively target cancer cells could be a very useful therapeutic, care must be taken to ensure that the technology does not also enable others to target healthy cells for malicious purposes. In the context of research on pathogens, while there are great benefits to be had in understanding how these organisms cause disease, the same research could inform others on how to make pathogens more dangerous. Accordingly, considerations of dual-use potential should be an important part of the research process.
Figure 5: (GHS Index 1.5.1b) The Index asks, “Is there legislation and/or regulation requiring oversight of research with especially dangerous pathogens, toxins, pathogens with pandemic potential and/or other dual-use research?”
As seen in Figure 5, despite the importance of dual-use risks, very few countries – Australia, Brazil, Canada, Thailand, Netherlands, Slovenia, Sweden, United Kingdom, and the United States – have legislation regarding dual-use oversight.
Antimicrobial Resistance Surveillance
In iGEM, we commonly use antibiotic selection to ensure that our parts have been successfully incorporated into the genome, and that our organism has been transformed. This form of selection is cheap, effective, and convenient. However, antimicrobial resistance (AMR) in general poses a larger threat to conventional medicine itself. Increasing prevalence of multiple antibiotic resistances in organisms like Staphylococcus aureus makes infections very difficult to treat and results in loss of life. Pathogens with multiple antibiotic resistances are proliferating faster than new antibiotics are being developed. The last lines of antibiotics are being threatened. Excessive and irresponsible use of antibiotics is the principal driver of this phenomenon. Globally, there are very few countries conducting environmental surveillance to monitor for AMR indicators in the environment.
Figure 6: (GHS Index 1.1.1c) The Index asks, “Does the government conduct environmental detection or surveillance activities (e.g., in soil, waterways) for antimicrobial residues or AMR organisms?”
According to Figure 6, the countries that do conduct surveillance for AMR characteristics are: Austria, France, Ireland, Japan, Jordan, Liechtenstein, Luxembourg, Netherlands, Sweden, Switzerland, Thailand, United Kingdom, and Vietnam.
DNA Synthesis Regulation
As seen in Figure 7, no countries have legislation or regulation requiring that DNA synthesis orders be screened to prevent the synthesis of pathogens or toxins. These types of regulations are important to prevent the inadvertent synthesis of toxins or pathogens, thereby exposing unprepared or untrained researchers to harm, or to prevent the deliberate synthesis of a toxin or pathogen for nefarious uses. Currently, DNA synthesis providers that are part of the International Gene Synthesis Consortium voluntarily screen the DNA that they synthesize at personal cost of time, money, and energy.
Figure 7: (GHS Index 1.5.2a) The Index asks, “Is there legislation and/or regulation requiring the screening of synthesized DNA (deoxyribonucleic acid) against lists of known pathogens and toxins before it is sold?”
Conclusion
There is a marked absence of regulations concerning biosafety and biosecurity around the world. Without national legislation and oversight, responsible conduct falls to individual scientists. iGEMers should carefully consider the biosafety and biosecurity aspects of their work and engage in their communities and nations to make sure that biosafety and biosecurity are priority issues moving forward. As iGEMers, we have a collective responsibility to make meaningful contributions to prevent synthetic biology from being misused.
References
World Health Organization. International Health Regulations (2005).
Nuclear Threat Initiative and Johns Hopkins Center for Health Security. Global Health Security Index (2019).
Via Unspalsh - Giammarco Boscaro