A Phoenix's View: iGEM Teams and Regenerative Medicine
Written By: Ahmed Basil Hussain
This article features iGEM Projects from 2012 WLC-Milwaukee, 2013 SYSU China, 2018 KAIT JAPAN, 2019 Munich, 2020 KCL_UK, 2021 KCL_UK, 2018 TecCEM, 2017 UCL, 2021 Cornell, 2019 NYU Shanghai’s 2019
Science fiction has shown us things from Deadpool’s rapid-regenerating limbs to Psycho-Pass’s interconnected, lab-grown human brains secretly governing a cyberpunk society. Unfortunately (or fortunately depending how you look at it), in reality, our biotechnological expertise on regeneration is comparatively very primitive. Regenerative medicine is a relatively small field in terms of currently applicable methods, but understanding it biologically is a wide, multidisciplinary, and active effort requiring insight from many disciplines of life sciences. Furthermore, understanding regenerative medicine mechanisms can aid in understanding cancer and immune mechanisms, due to the interplay between the three systems; stem cell proliferation often requires suppression of the immune response, since the body's natural immune response is to suppress uncontrolled division.
Image 1. Lab-grown brains in Psycho-Pass.
Stem Cells:
Shinya Yamanaka's discovery of induced pluripotent stem cells (iPSCs) in 2008 (created through the induced expression of 4 genes: octamer-binding transcription factor (Oct3/4), Kruppel-like factor 4 (Klf4), sex-determining region Y (Sox2), and c-Myc) marked a turning point in regenerative medicine. However, many restrictions and limitations around the use of the technology remain unresolved, including the relatively high levels of carcinogenesis in iPSC strains versus human embryonic stem cells (hESCs) and lack of optimized culture conditions for various iPSC strains. Further research into developmental pathways and infrastructure (i.e. specialized cell and tissue banks) is also just as important to progress the field.
Image 2. Patient-specific induced pluripotent stem cells (iPSCs) for the study and treatment of retinal degenerative diseases - Scientific Figure on ResearchGate.
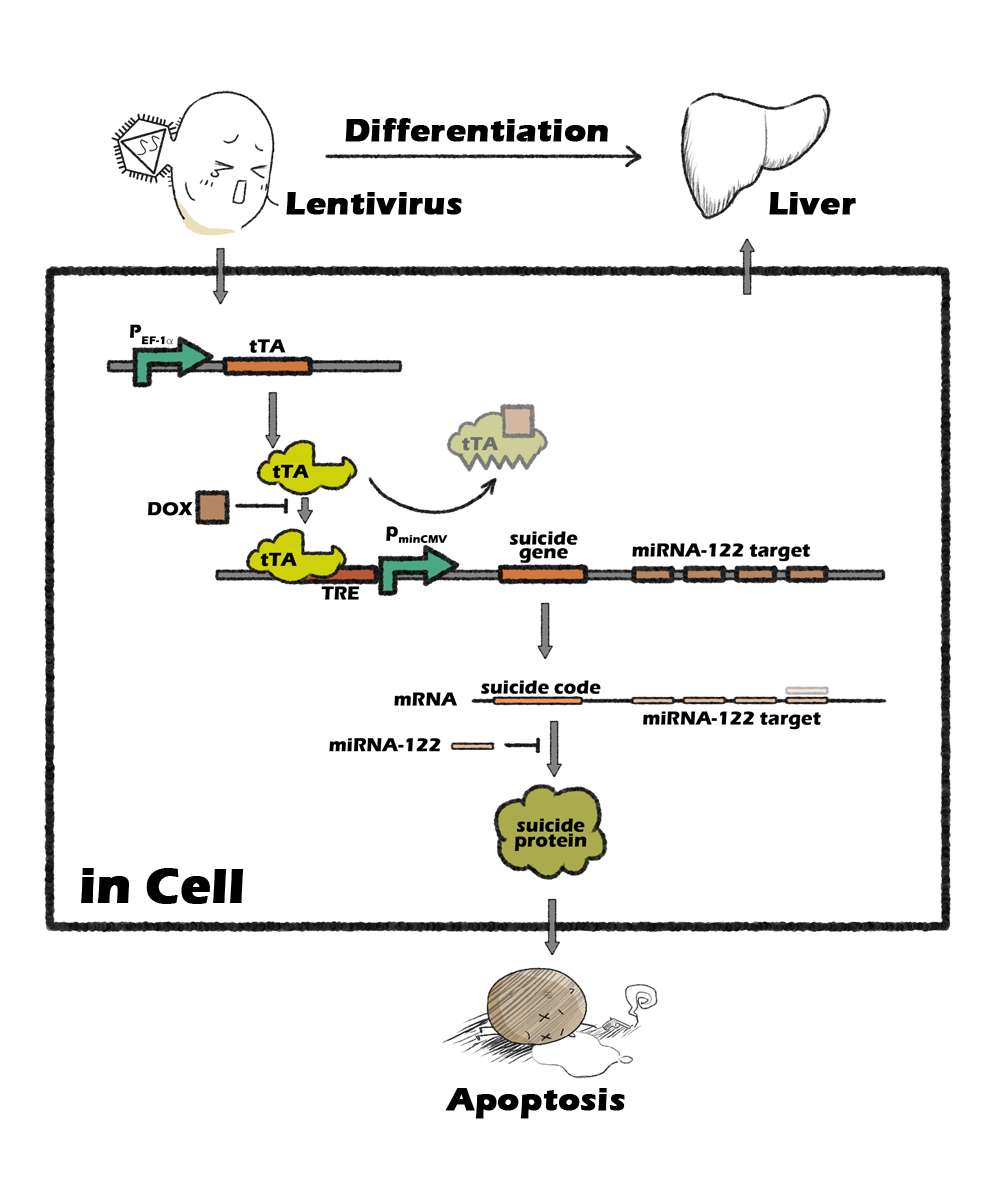
Several past iGEM teams have ventured to resolve some of those items. In regards to developmental pathways, 2012's WLC-Milwaukee team developed a genetic engineering platform, iDifferentiate, to "elucidate differentiation pathways of any cell type for which there is a known lineage-specific cis-regulatory element," in combination with their SAVE Assay, a customizable dual-plasmid system to visualize successfully differentiated cells. In regards to carcinogenesis control, SYSU China's Gold-medal winning 2013 device composed of an miRNA-122 sensor and tetracycline induction system allows the selection of hepatocytes from iPS differentiated cell mass. As for infrastructure, KAIT Japan developed a higher-survival alternative to cryopreservation in 2018, through an H₂S-induced state of hypometabolism followed by ATP depression using cystathionine γ-lyase (CTH) and regulating it through a quorum-sensing mechanism.
Image 3. SYSU China’s 2013 project overview.
A common misconception is that injected stem cells start self-replicating in vivo - however, the use of stem cells in therapy is more due to their conveniently timed-release, secreted molecules, which then induce the patient's own stem cells to replicate. However, there is ongoing research on the biophysical effects of scaffold materials on the self-renewal activity of stem cells in vitro, used in the development of miniature organs called organoids - which are promising drug-testing and research platforms that could reduce our reliance on animal models, and perhaps eventually pave the way to the development of lab-grown organs.
Organoids:
Organoids provide a platform for a more accurate testing of specific human processes, namely brain development, metabolism, and drug efficacy, but this does not mean that animal models are completely replaceable: C. elegans is often used for cell fate decision analysis due to its high genetic similarity to humans (even to human disease genes), as well as conserved cell death pathways; D. rerio is useful for large-scale mutagenesis and visual analysis of development through its transparent embryo; M. musculus has advantages in terms of available genetic tools and germline-competent embryonic stem cells. An important distinction, though, is that those animal model cell lines are inbred, which does not represent the role that genetic diversity plays in disease progression and drug response, which necessitates the development of human-specific model systems.
Image 4. Comparison of organoids with other model systems - Scientific Figure on Nature.
Challenges with organoid development include high variability in batches, lines, architecture, maturation, and diversification - which is a problem especially in developing organoid disease models, even using CRISPR-Cas9 isogenic controls. A potentially relevant gold-medal project from Munich’s 2019 iGEM team, nominated for best diagnostics project, developed a diagnostics platform for the Analysis of Living cells via Vesicular Export (ALiVE), which uses “bio-orthogonal RNA-adapters and modified membrane proteins with affinity tags to enable convenient purification of the exported RNA,” as well as “sensitive luciferase reporters to quantify vesicle secretion efficiency and collateral transfection.” The most obvious limitation though, is the lack of supply of nutrients and oxygen to the central regions of the tissue, which along with the lack of innervation, vascularization, and immunization affects our ability to recapitulate the disease process, especially given the lack of disease models. A promising fix for this is the co-culture of organoids with mesenchymal and/or immune cell populations, which takes us into the realm of organs-on-a-chip and 3D bioprinting.
Image 5. Munich’s 2019 project overview.
3D Bioprinting:
Biophysical properties such as shear strength and elasticity are important considerations in modern biotechnological applications; building organoid architecture utilizes various biomaterials and chemistries, such as hydrogels and carbon nanotubes, which form the scaffold from which the stem cell base can replicate and can help induce angiogenesis for perfusion of the growing organoid. There are multiple approaches by which in-vitro organoid perfusion is achieved: cardiomyocyte seeding onto the bioprinted scaffold, printing vascularized tissue by processing cells’ own matrix into a hydrogel encapsulating the reprogrammed cells, printing surrounding tissue and externally perfusing through micro-molded agarose networks, or inducing self-organization of endothelial cell co-culture using angiogenic growth factors and with the help of microfluidics (essential part of organ-on-a-chip technology). The latter creates in-vitro organoids with functionality closest to in-vivo transplanted organoids in terms of angiogenesis, morphology, and permeability - however, the use of microfluidics also disturbs the self-organization of the organoids due to the lack of versatility required to adapt to real-time organoid changes and due to the physical limitations of a microfluidic system compared to a fully functioning cardiac system (e.g. vasomotor tone and vascular resistance control).
Image 6. The procedure of endothelialized myocardium fabrication using the 3D bio-printing strategy - Scientific Figure on Frontiers in Bioengineering and Biotechnology.
iGEMer contributions in this area include 2020’s best therapeutics project, KCL_UK’s polycaprolactone scaffold incorporating a “synthetic mussel-foot protein based bioadhesive coating, to encourage axonal attachment” as a potential regenerative therapy for spinal cord injury and 2018’s best therapeutics nomination from TecCEM using “a multi-glycopeptide scaffold and the recombinant growth factor Leptin B to induce fibroblast proliferation” to enhance tissue regeneration for second degree burn patients. There are efforts to develop organoid-on-a-chip technology, synergizing the benefits of both microfluidic systems and self-organizing systems. However, there is a great deal of biological knowledge not yet simulatable regarding the exact microenvironment and extracellular matrices of developing tissues and a lack of engineering knowledge in creating dynamic systems able to respond to biological cues in real-time. This is where the field of bioelectricity, developed by Michael Levin and his team at Tufts University, plays a role.
Image 7. KCL UK’s 2020 project overview.
Bioelectricity:
As Levin states, bioelectricity is a field as old as biochemistry and microbiology, but unlike the other two can only be studied in living tissue. The field focuses on the voltage gradients of cell membranes and their effects on cell and tissue physiology, and Levin proposes that not only are bioelectrical cues markers for genetic change, but can even override genetic processes. An example of this was shown in one of his experiments, where the oncogenic effects of a mutated p53 gene were blocked through forcing the cell’s ion channels open and thus maintaining a shared internal environment and communication channel with surrounding cells. Another of his experiments mapped the voltage pattern for developmental eye formation and induced that pattern in gut cells, through the introduction of RNA coding for a specific ion channel, with the surprising result of eye development within the gut despite the cells’ different germ layer origins. Other experiments include planarian bioelectric head modification and the creation of “xenobots” from developing tadpole skin cells. This field has big implications on the fundamentals of development, cancer, anatomy, evolution, and regeneration - and could fill in gaps in understanding the complexities of extracellular matrix function as well as the basics of cell manipulation.
Image 8. Endogenous Bioelectric Prepatterns - Scientific Figure on Foresight Institute.
There are many adjacent technologies that could help develop the field of bioelectricity - for example, biochemical optimization of the voltage dyes used to map cells’ voltage gradients or engineering of novel devices and algorithms to help map out other aspects of the cell (i.e. spatial biology; see Human Cell Atlas). From iGEM, those include UCL’s 2017 optogenetic tools for “inter-cell adhesion and directed gene expression of mammalian cells for tissue generation;” Cornell’s 2021 collagen scaffold utilizing engineered Scl2 proteins for customizable arrangements of integrin and fibronectin throughout the hydrogel, allowing for micro-control of spatial distribution of various cell-lines; and NYU Shanghai’s 2019 project using fish scales’ piezoelectric properties to allow for initiation of electrical signaling within a tissue scaffold. The latter holds promise with the recent 2022 discovery of easy production of carbon nano-onions, a material that can be used for bioimaging and sensing, through the microwaving of fish scales.
Image 9. UCL’s 2017 project overview.
As mentioned earlier, the field of regenerative medicine is likely to go through big shifts soon, and multidisciplinary efforts will be required to develop a complete picture of the various pieces of research currently in progress and yet to come. But, as long as curiosity for answering those research questions is maintained, a regenerative future is in our hands.