Common Challenges In Your Bio-Entrepreneurship Journey and How To Overcome Them
This blogpost is created by the Policy Research project for the iGEM and SynBio Community
In its 20-year journey, iGEM has witnessed remarkable growth and played a pivotal role in shaping the landscape of synthetic biology. As we celebrate two decades of iGEM's impact, it's impressive that over 250 startups have emerged from this dynamic ecosystem. These innovative ventures have brought to life many groundbreaking ideas, driving us closer to a brighter future in synthetic biology.
The trajectory of synthetic biology is undeniably promising, and more iGEM projects will embark on the thrilling journey of establishing themselves as entrepreneurial ventures. This blog post serves as a comprehensive handbook, offering insights and strategies to navigate the challenges to guide iGEMers through the intricate path of entrepreneurship. Whether it's surmounting scientific complexities, securing vital funding, navigating regulatory pathways, protecting intellectual property, or ensuring market acceptance and validation, this guide is your compass for charting a successful course in the vibrant world of synthetic biology.
Here’s a quick overview of a “typical” bio-entrepreneurship roadmap;
How does a bio-entrpreneurship roadmap look like?
Embarking on your entrepreneurship journey, where your innovative ideas turn into thriving ventures, requires a well-structured roadmap. This comprehensive guide can be broken down into several key phases. It all begins with an initiation, where the entrepreneurial spirit takes root through idea generation and validation, followed by the critical step of team building to bring those ideas to life. The next phase, preparation, involves conducting thorough market research, crafting a compelling business plan, strategically considering intellectual property protection, and honing pitching and networking skills. Once the groundwork is solid, the execution phase comes into play. Here, entrepreneurs dive into prototyping and testing their concepts, navigating the intricate world of regulatory files and applications. Finally, the grand launch phase arrives, marked by the commercialization and scaling of the venture, underpinned by a commitment to continuous learning and adaptation to ensure long-term success in the ever-evolving field of bio-entrepreneurship. This roadmap guides aspiring bio-entrepreneurs, helping them navigate the complex journey from concept to market, unlocking the immense potential of the biotech industry.
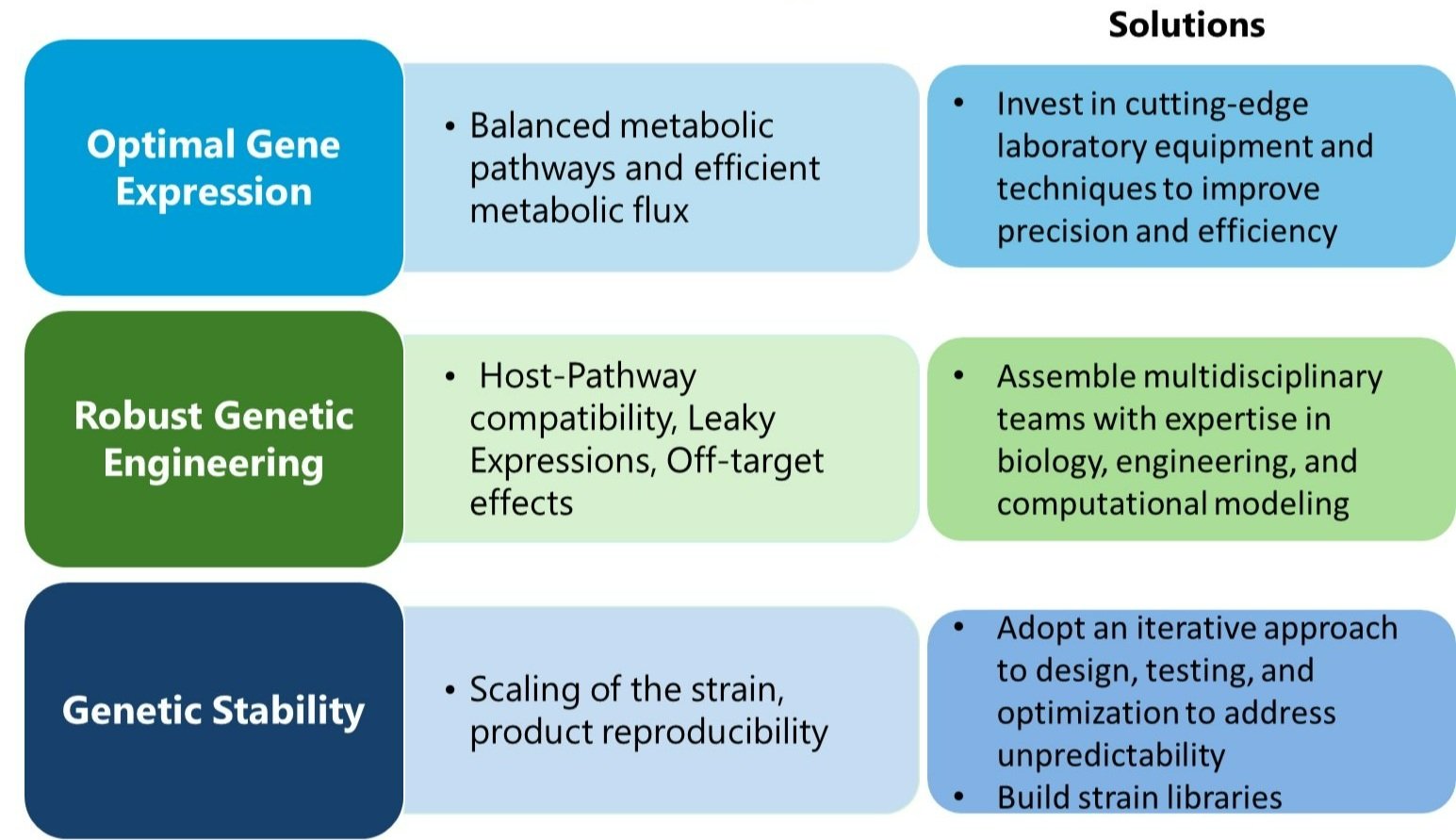
Once you have started working on your products, you may encounter many challenges, and here we are giving you a concise breakdown of those challenges:
Scientific and technical challenges for bio-startup
Navigating the realm of synthetic biology and biotechnology involves grappling with intricate scientific and technical challenges. This involves dealing with the unpredictability of living systems, optimizing metabolic pathways, and ensuring the stability and consistency of genetically engineered organisms. Understanding and addressing these challenges are vital for successfully developing and applying synthetic biology innovations.
Funding and investment challenges for Bio-startup
Securing funding in the biotechnology sector, particularly synthetic biology, presents a unique set of challenges. Investors may perceive these ventures as high-risk due to the complex nature of the field and more extended return on investment timelines. Overcoming these funding hurdles often requires a combination of education, risk mitigation, strategic partnerships, and diverse funding sources.
Regulatory Challenges for Bio-startup
Regulatory requirements are paramount in synthetic biology, encompassing biosafety, ethical concerns, and environmental impact. Navigating the complex and evolving regulatory landscape for genetically modified organisms (GMOs) and biotechnology products is critical. Ensuring compliance with these regulations is a multifaceted task that involves early engagement with regulatory agencies, risk assessments, and ethical discussions.
Intellectual Property Process
When creating a solid business strategy, intellectual property and protecting your innovative ideas are essential to your success. The patent application procedure involves drafting detailed descriptions of claims and often working with regulatory experts. This process requires strategic decision-making regarding jurisdiction, understanding prior art, and engaging legal professionals well-versed in biotech patent law.
Here’s a breakdown of a patent journey and some critical information on the procedure;
Invention Disclosure (0-6 months): Before filing a patent application, the inventor(s) should document the invention thoroughly, including its technical details, novelty, and potential applications.
Prior Art Search (1-2 months): Conduct a prior art search to identify existing patents, publications, or technologies similar to the invention. This helps assess novelty and patentability.
Prepare a Patent Application (2-6 months): Draft a detailed patent application, including the title, abstract, and summary. It must have a detailed description of the invention, including drawings or diagrams, claims defining the scope of protection sought, background, detailed description, and examples. Inventors must provide any supporting data or evidence in their application.
Choose a Jurisdiction: Decide in which countries or regions you want to seek patent protection. Consider factors such as market potential and budget.
File a Priority Application (your patent will be valid from this day): The priority application, often a provisional patent application, establishes a priority date for your invention. You must file a formal, non-provisional application 12 months from the priority date in many countries. At this stage, you can file for an international patent application. This is called a PCT (Patent Cooperation Treaty) application, which gives your patent a protection status in multiple countries.
Examination and Publication (18-36 months): After filing, the patent office examines the application to ensure it meets patentability criteria (novelty, non-obviousness, utility, enablement, etc.). The patent office may issue office actions requesting amendments or additional information. Once the application is deemed allowable, it is published, typically 18-36 months from the priority date.
Responses to Office Actions: You must respond with amendments or arguments if the patent office issues “office actions.” The main patent office where you made your initial application will give you specific deadlines and requests to attend.
Grant and Publication: The patent is granted if the patent office agrees that all requirements are met. Publication of the granted patent typically occurs shortly after the grant.
Maintenance Fees (yearly until the patent expires): To keep the patent in force, you must pay maintenance fees at specified intervals, which can vary by country.
Patent Expiry (typically 20 years from filing): In most cases, patents are valid for 20 years from the filing date (or the priority date, if applicable). Once the patent expires, the invention enters the public domain, and others can use it freely.
Market Entry and Adoption Challenges for a biostartup
Let’s say you have a patent application and a successful product; now, you want to launch your product. There are still some challenges you might face on the market end.
Introducing synthetic biology products into the market demands technical skillfulness and the ability to convince potential customers and investors of the feasibility and value of these innovations. Achieving market acceptance often involves demonstrating consistent and reproducible performance, tailoring products to industry-specific needs, and garnering early user feedback through prototypes and iterative development cycles. These steps are pivotal for successful market adoption.
Are you an iGEMer and do you want to start a bio-startup? Are you looking for resources to help in your bio-entrepeneurship journey? Are you a biofounder and do you want to share your story? Check out the iGEM Startups!